Buloxibutid (C21) – IPF
Buloxibutid (C21) is a first-in-class orally available low molecular weight angiotensin II type 2 receptor agonist (ATRAG).
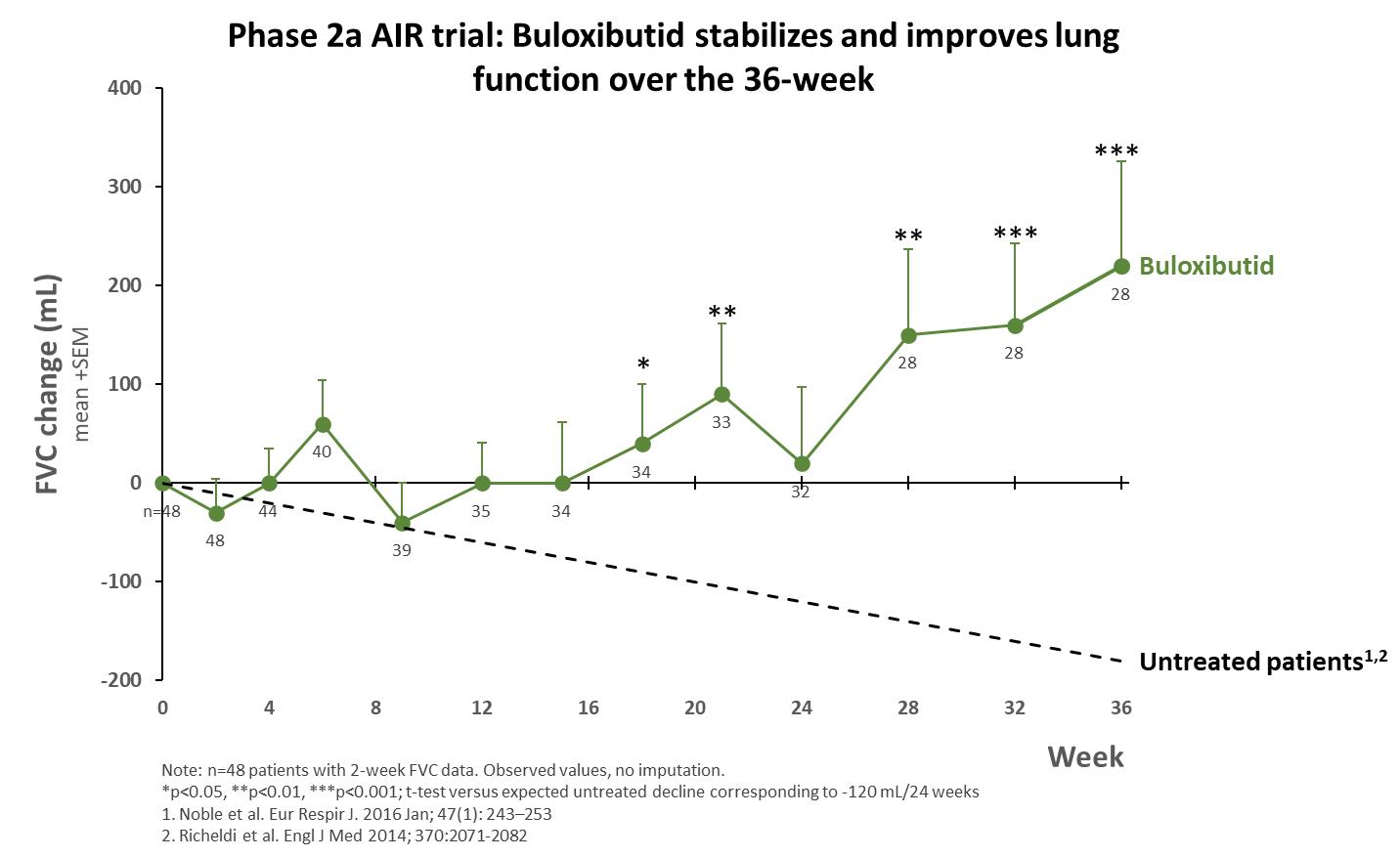
The novel and multimodal mechanism of action of buloxibutid targets the underlying fibrosis in IPF by stimulating the protective arm of the renin-angiotensin system. Consequently, there is an upstream effect in terms of promoting alveolar repair and maintenance of alveolar integrity thereby reducing fibrosis formation, stabilizing disease, and increasing lung capacity.
The AIR trial is a phase 2a multi-center, open label, single arm 36-week trial evaluating the safety and efficacy of buloxibutid in patients with IPF.
Read more about the AIR trial: Clinicaltrials.gov
Buloxibutid is now being evaluated in the global, randomized, 52-week Phase 2b ASPIRE trial.